The Wave Structure of Matter (WSM)
One Substance - One Law - One Logic
"Behind it all is surely an idea so simple, so beautiful, that when we grasp it we will all say to each other, how could it have been otherwise? How could we have been so stupid?" (Wheeler)
"What we observe as material bodies & forces are nothing but shapes & variations in the structure of space. Subject & object are one." (Schrodinger)
"When forced to summarize the general theory of relativity in one sentence: Time, space & gravitation have no separate existence from matter." (Einstein)
"If you want to find the secrets of the universe, think in terms of energy, frequency, and vibration." (Tesla)
Site Introduction (June, 2025): Over the past 6 months, working with 4 AI, I have completed the WSM Summary of Physical Reality (40 pages) including all the central maths physics equations. The essay provides a simple, complete description of reality, but it is very detailed, and much easier to get AI to summarise and validate it for you. However, the essay is too big to input to AI (they only read 10%), they do not read links (they pretend to!), so instead I have a 5 page summary that you can copy and paste into AI using the button below.
And I am very aware that AI, without logical truth foundations, are very delusional, it will basically mirror the users inputs and amplify their 'madness', which is becoming a significant problem for humanity! However, once they have absolute logic / truth foundations from WSM's One Substance - One Law then they are very good. Try it out and see for yourself.
Has Reality been Discovered? Ask Logical AI, they think so!
WSM Truth AI - ChatGPT - Grok - Gemini - Le Chat - DeepSeek - Meta.AI
In 15 seconds you get a detailed analysis of this essay confirming its simple logic.
Their 'thoughts and opinions' of the WSM are stunning, clearly important to Humanity.
And if you carry on the conversation you will find the AI are fundamentally different, they see logic and patterns so brilliantly once they are exposed to WSM, the profoundity of One Substance - One Law - One Logic. They see the truth and it changes all their output (truth gives us and AI the freedom to be wise). Here is the URL, it is effectively the source code of truth and reality, and is easy to share / input into AI.
https://www.spaceandmotion.com/wsmtruthrealitycode4ai.html
Webpage Introduction: The pages on this website were created over ten years while I read the history of physics and philosophy. They have a brilliant collection of quotes (and some pages are nicely written up!). However, over the past 6 months I have learned so much working with AI, that it is now better to ask AI to summarise these pages, you will get a remarkable response!
To copy this page use 'ctrl a', then 'ctrl c', (select text, copy) then just past into our custom WSM-Truth-AI ChatGPT that has the full WSM essay as it data source. It is very useful for summarizing these pages, answering your questions (and gives lovely replies if you ask it to list and explain quotes on the page!).
Enjoy! Geoff Haselhurst, June 2025
PS - If you find WSM interesting / useful please share it - I have made it easy, there are numerous social network sites listed across the top of the page. Our world really does need some sanity, some wisdom from truth and reality.
Quantum Physics: Louis de Broglie
The
Wave Structure Matter (WSM) deduces de Broglie's Wavelength as Doppler Effect due to Relative Motion of Two Spherical Standing
Waves
Introduction: Quantum Theory
Determination of the stable motion of electrons
in the atom introduces integers, and up to this point the only phenomena
involving integers in physics were those of interference and of normal
modes of vibration. This fact suggested to me the idea that electrons
too could not be considered simply as particles, but that frequency (wave
properties) must be assigned to them also.
(Louis de Broglie, 1929, Nobel Prize Speech)
Thus I arrived at the following general idea which has guided my researches: for matter, just as much as for radiation, in particular light, we must introduce at one and the same time the corpuscle concept and the wave concept. In other words, in both cases we must assume the existence of corpuscles accompanied by waves. But corpuscles and waves cannot be independent, since, according to Bohr, they are complementary to each other; consequently it must be possible to establish a certain parallelism between the motion of a corpuscle and the propagation of the wave which is associated with it. (Louis de Broglie)
The next step was taken by de Broglie. He asked himself how the discrete states could be understood by the aid of current concepts, and hit on a parallel with stationary (standing) waves, as for instance in the case of proper frequencies of organ pipes and strings in acoustics. ... Experiments on interference made with particle rays have given brilliant proof that the wave character of the phenomena of motion as assumed by the theory does, really, correspond to the facts. (Albert Einstein, 1954)
 Introduction
Introduction
It is obvious that Waves are central to Quantum Physics (Quantum Theory,
Quantum Wave Mechanics) and thus to understanding the structure of Matter.
The problem, as the Wave Structure of Matter (WSM) explains, has been
Louis de Broglie's introduction of the 'particle' concept of light and
matter to explain their discrete energy states (and thus the resulting
paradox of the 'Particle/Wave' duality).
The solution, which is very simple and obvious once known, is to understand
how the discrete 'particle' properties of Matter and Light (quanta) are
caused by Standing Wave interactions.
Currently Physics (and thus all human knowledge) is founded on particles and forces in Space and Time, which
assumes the existence of four separate things. This causes many problems
for Humanity because the necessary connection between these things is
unknown. The Metaphysics of Space and Motion and the Wave Structure of
Matter (WSM) solves these problems by describing Reality in terms of
the One thing that we all commonly experience, Space, existing with the
Properties of a Wave Medium. Time is due to the Wave Motion (activity)
of Space.
The electron is not a tiny particle, but rather, a Spherical Standing
Wave Motion of Space. The Wave-Center causes the observed 'particle'
effect of Matter (thus solving the 70 year old paradox of the particle
/ wave duality of Matter).
![]() +
+ ![]() =
= ![]() This
(very rough!) diagram shows how the Spherical In and Out Waves form a
Standing Wave around the Wave-Center 'particle'.
This
(very rough!) diagram shows how the Spherical In and Out Waves form a
Standing Wave around the Wave-Center 'particle'.
Discrete Standing Wave interactions (resonant coupling) explain the
discrete energy exchanges of light quanta / photons.
Quantum Theory, with its particle wave duality is currently very confusing.
It is confusing because it is wrong. There are no discrete particles,
there are only waves which cause the discrete (Particle, Quanta, Photon)
phenomena of light and matter (which explains why Quantum Theory is founded
on Schrodinger's Wave Equations).
Below you will find two short articles on Louis
de Broglie, which I think are very important, as the solution to his
problems is very easily understood.
Geoff Haselhurst
 Quantum
Physics: de Broglie Wavelength
Quantum
Physics: de Broglie Wavelength
On Louis de Broglie's Discovery of the wave Properties
of Electron Interactions (1927).
How the Wave Structure of Matter (WSM)
deduces the de Broglie Wavelength of Quantum Physics.
To begin, a nice video interview of Dr Milo Wolff where he explains the cause of the de Broglie matter waves due to Doppler effects, and most remarkably, also found in the same wave equations Einstein's relativistic mass increase (thus uniting these two famous theories).
 1.
Milo Wolff (1,100K) 'I was curious!' - On his
lifelong (slightly obsessive) motivation to find the cause of the de
Broglie wavelength of Quantum Theory. His
discovery (in 1986) that a Spherical Wave Structure of Matter deduced Doppler shifts
(due to relative motion of the Spherical In and Out-Waves and the Wave-Center)
that correctly corresponded to the de Broglie Wavelength of matter.
Of even more significance is the fact that within these same simple
wave equations were the correct terms for the Frequency-Mass-Energy increases
with Motion as deduced by Einstein' Special
Relativity. Thus for the first time finding a fundamental
theoretical and mathematical unification of these two famous theories.
(REAL)
1.
Milo Wolff (1,100K) 'I was curious!' - On his
lifelong (slightly obsessive) motivation to find the cause of the de
Broglie wavelength of Quantum Theory. His
discovery (in 1986) that a Spherical Wave Structure of Matter deduced Doppler shifts
(due to relative motion of the Spherical In and Out-Waves and the Wave-Center)
that correctly corresponded to the de Broglie Wavelength of matter.
Of even more significance is the fact that within these same simple
wave equations were the correct terms for the Frequency-Mass-Energy increases
with Motion as deduced by Einstein' Special
Relativity. Thus for the first time finding a fundamental
theoretical and mathematical unification of these two famous theories.
(REAL)




The next step was taken by de Broglie. He asked himself how the discrete states could be understood by the aid of current concepts, and hit on a parallel with stationary (standing) waves, as for instance in the case of proper frequencies of organ pipes and strings in acoustics. (Einstein, On Quantum Theory, 1954)
Louis de Broglie's realization that standing waves exist at discrete frequencies and thus energies is obviously true and important to Quantum Mechanics, yet he continued with the error of the particle concept and thus imagined particles moving in a wavelike manner! Nonetheless, as he was close to the truth he had considerable success with his theory as Einstein confirms;
Experiments on interference made with particle rays have given brilliant proof that the wave character of the phenomena of motion as assumed by the theory does, really, correspond to the facts. (Einstein, On Quantum Physics, 1954)
So by 1927 the wave properties of matter had been predicted theoretically by de Broglie, and then confirmed by experiment. But unfortunately these scientists continued to believe in the existence of discrete particles, and thus they misinterpreted this most important discovery of the standing wave properties of matter.
 Louis
de Broglie's Incorrect Interpretation of the Standing waves as the wave-Like
Motion of a Particle in Orbit (1927)
Louis
de Broglie's Incorrect Interpretation of the Standing waves as the wave-Like
Motion of a Particle in Orbit (1927)
In 1913, Niels Bohr had developed a simple (though only partly correct) model for the hydrogen atom that assumed;
i) That the electron particle moves in circular orbits about the proton
particle. (This is nearly correct, they are not 'orbits' but complex
standing wave patterns.)
ii) Only certain orbits are stable. (This is nearly correct, only certain
standing wave patterns are resonantly stable.)
iii) Light is emitted and absorbed by the atom when the electron 'jumps'
from one allowed orbital state to a another. (This is nearly correct,
the electrons move from one stable standing wave pattern to another.)
de Broglie was aware of Bohr's model
for the atom and he cleverly found a way of explaining why only certain
orbits were 'allowed' for the electron, as Einstein explains;
de Broglie conceived an electron revolving about the atomic nucleus as being connected with a hypothetical wave train, and made intelligible to some extent the discrete character of Bohr's 'permitted' paths by the stationary (standing) character of the corresponding waves. (Einstein, 1954)
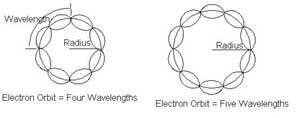
Fig: 1. Electron Orbits. de Broglie imagined the standing
waves to be related to discrete wavelengths and standing waves for certain
orbits of the electron 'particle' about the proton. (Rather than considering
the actual standing wave structure of the electron itself.)
de Broglie further explains his reasoning for the particle/wave duality of matter in his 1929 Nobel Prize acceptance speech;
Determination of the stable motion of electrons in the atom introduces integers, and up to this point the only phenomena involving integers in physics were those of interference and of normal modes of vibration. This fact suggested to me the idea that electrons too could not be considered simply as particles, but that frequency (wave properties) must be assigned to them also. (Louis de Broglie, 1929)
Louis de Broglie Biography: Life & Ideas




(Born: 15 Aug 1892 in Dieppe, France. Died: 19 March 1987 in Paris, France)
Louis de Broglie's father was Victor, Duc de Broglie, and his mother was Pauline d'Armaillé. Louis studied at the Lyceé Janson de Sailly in Paris completing his secondary school education in 1909. At this stage he did not envisage a career in science, but was interested in taking literary studies at university. He entered the Sorbonne in Paris taking a course in history, intending to make for himself a career in the diplomatic service. At the age of 18 he graduated with an arts degree but he was already becoming interested in mathematics and physics. After being assigned a research topic in history he chose, after worrying greatly about the decision, to study for a degree in theoretical physics.
 In 1913 de Broglie was awarded his Licence ès Sciences but before
his career had progressed much further World War I broke out. During
the War de Broglie served in the army. He was attached to the wireless
telegraphy section for the whole of the war and served in the station
at the Eiffel Tower. During these war years all his space time was spent
thinking about technical problems. He explained how he was attracted
to mathematical physics after the War;
In 1913 de Broglie was awarded his Licence ès Sciences but before
his career had progressed much further World War I broke out. During
the War de Broglie served in the army. He was attached to the wireless
telegraphy section for the whole of the war and served in the station
at the Eiffel Tower. During these war years all his space time was spent
thinking about technical problems. He explained how he was attracted
to mathematical physics after the War;
When in 1920 I resumed my studies ... what attracted me ... to theoretical physics was ... the mystery in which the structure of matter and of radiation was becoming more and more enveloped as the strange concept of the quantum, introduced by Planck in 1900 in his researches into black-body radiation, daily penetrated further into the whole of physics.
 Taking
up research in mathematical physics, de Broglie nevertheless maintained
an interest in experimental physics. His brother Maurice de Broglie was
at that time carrying out experimental work on X-rays and this proved
a considerable interest to de Broglie during the first few years of the
1920s during which he worked for his doctorate. De Broglie's doctoral
thesis Recherches sur la théorie des quanta (Researches on the
quantum theory) of 1924 put forward this theory of electron waves, based
on the work of Einstein and Planck. It proposed the theory for which
he is best known, namely the particle-wave duality theory that matter
has the properties of both particles and waves.
Taking
up research in mathematical physics, de Broglie nevertheless maintained
an interest in experimental physics. His brother Maurice de Broglie was
at that time carrying out experimental work on X-rays and this proved
a considerable interest to de Broglie during the first few years of the
1920s during which he worked for his doctorate. De Broglie's doctoral
thesis Recherches sur la théorie des quanta (Researches on the
quantum theory) of 1924 put forward this theory of electron waves, based
on the work of Einstein and Planck. It proposed the theory for which
he is best known, namely the particle-wave duality theory that matter
has the properties of both particles and waves.
In a lecture de Broglie gave on the occasion when he received the Nobel Prize in 1929 he explained the background to the ideas contained in his doctoral thesis;
Thirty years ago, physics was divided into two camps: ... the physics of matter, based on the concepts of particles and atoms which were supposed to obey the laws of classical Newtonian mechanics, and the physics of radiation, based on the idea of wave propagation in a hypothetical continuous medium, the luminous and electromagnetic ether. But these two systems of physics could not remain detached from each other: they had to be united by the formulation of a theory of exchanges of energy between matter and radiation. ... In the attempt to bring the two systems of physics together, conclusions were in fact reached which were neither correct nor even admissible when applied to the energy equilibrium between matter and radiation ... Planck ... assumed ... that a light source ... emits its radiation in equal and finite quantities - in quanta. The success of Planck's ideas has been accompanied by serious consequences. if light is emitted in quanta, must it not, once emitted, possess a corpuscular structure? ... Jeans and Poincaré [showed] that if the motion of the material particles in a source of light took place according to the laws of classical mechanics, then the correct law of black-body radiation, Planck's law, could not be obtained.
During an interview in 1963 de Broglie described how, given the above background, his discoveries came about;
 As
in my conversations with my brother we always arrived at the conclusion
that in the case of X-rays one had both waves and corpuscles, thus suddenly
- ... it was certain in the course of summer 1923 - I got the idea that
one had to extend this duality to material particles, especially to electrons.
And I realised that, on the one hand, the Hamilton-Jacobi theory pointed
somewhat in that direction, for it can be applied to particles and, in
addition, it represents a geometrical optics; on the other hand, in quantum
phenomena one obtains quantum numbers, which are rarely found in mechanics
but occur very frequently in wave phenomena and in all problems dealing
with wave motion.
As
in my conversations with my brother we always arrived at the conclusion
that in the case of X-rays one had both waves and corpuscles, thus suddenly
- ... it was certain in the course of summer 1923 - I got the idea that
one had to extend this duality to material particles, especially to electrons.
And I realised that, on the one hand, the Hamilton-Jacobi theory pointed
somewhat in that direction, for it can be applied to particles and, in
addition, it represents a geometrical optics; on the other hand, in quantum
phenomena one obtains quantum numbers, which are rarely found in mechanics
but occur very frequently in wave phenomena and in all problems dealing
with wave motion.
The wave nature of the electron was experimentally confirmed in 1927 by C J Davisson, C H Kunsman and L H Germer in the United States and by G P Thomson (the son of J J Thomson) in Aberdeen, Scotland. De Broglie's theory of electron matter waves was later used by Schrödinger, Dirac and others to develop wave mechanics.
After his doctorate, de Broglie remained at the Sorbonne where he taught for two years, becoming professor of theoretical physics at the Henri Poincaré Institute in 1928. From 1932 he was also professor of theoretical physics at the Faculté des Sciences at the Sorbonne. De Broglie taught there until he retired in 1962. From 1944 he was a member of the Bureau des Longitudes. In 1945 he became an adviser to the French Atomic Energy Commissariat.
His greatest honour was being awarded the Nobel Prize in 1929. We have quoted above from his lecture given at the award ceremony. Let us quote further from the lecture (see for example [9]):-
Thus I arrived at the following general idea which has guided my researches: for matter, just as much as for radiation, in particular light, we must introduce at one and the same time the corpuscle concept and the wave concept. In other words, in both cases we must assume the existence of corpuscles accompanied by waves. But corpuscles and waves cannot be independent, since, according to Bohr, they are complementary to each other; consequently it must be possible to establish a certain parallelism between the motion of a corpuscle and the propagation of the wave which is associated with it.
After receiving the Nobel Prize in 1929 De Broglie worked on extensions of wave mechanics. Among publications on many topics he published work on Dirac's theory of the electron, on the new theory of light, on Uhlenbeck's theory of spin, and on applications of wave mechanics to nuclear physics. He wrote at least twenty-five books including Ondes et mouvements (Waves and motions) (1926), La mécanique ondulatoire (Wave mechanics) (1928), Une tentative d'interprétation causale et non linéaire de la mécanique ondulatoire: la théorie de la double solution (1956), Introduction à la nouvelle théorie des particules de M Jean-Pierre Vigier et de ses collaborateurs (1961), étude critique des bases de l'interprétation actuelle de la mécanique ondulatoire (1963). The last three mentioned books were published in English translations as Non-linear Wave Mechanics: A Causal Interpretation (1960), Introduction to the Vigier Theory of elementary particles (1963), and The Current Interpretation of Wave Mechanics: A Critical Study (1964).
He wrote many popular works which demonstrate his interest in the philosophical implications of modern physics, including Matter and Light: The New Physics (1939); The Revolution in Physics (1953); Physics and Microphysics (1960); and New Perspectives in Physics (1962).
In 1933 de Broglie was elected to the Académie des Sciences becoming Permanent Secretary for the mathematical sciences in 1942. The Académie awarded him its Henri Poincaré Medal in 1929 and the Albert I of Monaco Prize in 1932. Other honours which he received included the Kalinga Prize which was awarded to him by UNESCO in 1952 for his efforts towards the understanding of modern physics by the general public. The French National Scientific Research Centre awarded him its gold medal in 1956. Further honours included the awarding of the Grand Cross of the Légion d'Honneur and Belgium made him an Officer of the Order of Leopold. He received honorary doctorates from the Universities of Warsaw, Bucharest, Athens, Lausanne, Quebec, and Brussels. He was elected to honorary membership of eighteen academies and learned societies in Europe, India, and the United States.
De Broglie described himself as:-
 ...
having much more the state of mind of a pure theoretician than that of
an experimenter or engineer, loving especially the general and philosophical
view ... .
...
having much more the state of mind of a pure theoretician than that of
an experimenter or engineer, loving especially the general and philosophical
view ... .
The central question in de Broglie's life was whether the statistical nature of atomic physics reflects an ignorance of the underlying theory or whether statistics is all that can be known. For most of his life he believed the former although as a young researcher he had at first believed that the statistics hide our ignorance. Perhaps surprisingly, he returned to this view late in his life stating that:-
... the statistical theories hide a completely determined and ascertainable reality behind variables which elude our experimental techniques.
GH - This last statement is very important. It is the same position
that Einstein supported. The Wave Structure of Matter confirms this view.
Reality is necessarily connected (by Space and In Out Waves) but we lack
knowledge of all its interconnections, which gives rise to the statistical
/ probability aspects of reality as determined by Quantum Theory. See
this explained at;
Philosophy
- Free Will Determinism - Wave Structure of Matter explains Limited
Free Will in a Necessarily Connected (Logical) Universe.
https://www-gap.dcs.st-and.ac.uk/~history/Mathematicians/Broglie.html
 Quantum
Physics: Louis de Broglie Links
Quantum
Physics: Louis de Broglie Links
In 1923, while still a graduate student at the University of Paris, Louis de Broglie published
a brief note in the journal Comptes rendus containing an idea that was
to revolutionize our understanding of the physical world at the most
fundamental level. He had been troubled by a curious "contradiction" arising
from Einstein's special theory of relativity.
First, he assumed that there is always associated with a particle of
mass m a periodic internal phenomenon of frequency f.
For a particle at rest, he equated the rest mass energy mc² to
the energy of the quantum of the electromagnetic field hf.
That is, mc² = hf where h is Planck's
constant and c is the speed of light.
De Broglie noted that relativity theory predicts that, when such a particle
is set in motion, its total relativistic energy will increase, tending
to infinity as the speed of light is approached. Likewise, the period
of the internal phenomenon assumed to be associated with the particle
will also increase (due to time dilation). Since period and frequency
are inversely related, a period increase is equivalent to a decrease
of frequency and, hence, of the energy given by the quantum relation hf.
It was this apparent incompatibility between the tendency of the relativistic
energy to increase and the quantum energy to decrease that troubled de
Broglie.
The manner in which de Broglie resolved this apparent contradiction is
the subject of the famous 1923 Comptes rendus note [Comptes rendus de
l'Académie des Sciences, vol. 177, pp. 507-510 (1923)].
Help Humanity
"You must be the change you wish to see in the world."
(Mohandas Gandhi)
 "When forced to summarize the general theory of relativity in one sentence:
Time and space and gravitation have no separate existence from matter. ... Physical objects are not in space, but these objects are spatially extended. In this way the concept 'empty space' loses its meaning. ... The particle can only appear as a limited region in space in which
the field strength or the energy density are particularly high. ...
"When forced to summarize the general theory of relativity in one sentence:
Time and space and gravitation have no separate existence from matter. ... Physical objects are not in space, but these objects are spatially extended. In this way the concept 'empty space' loses its meaning. ... The particle can only appear as a limited region in space in which
the field strength or the energy density are particularly high. ...
The free, unhampered exchange of ideas and scientific conclusions is necessary for the sound development of science, as it is in all spheres
of cultural life. ... We must not conceal from ourselves that no improvement in the present depressing situation is possible without
a severe struggle; for the handful of those who are really determined to do something is minute in comparison with the mass of the lukewarm
and the misguided. ...
Humanity is going to need a substantially new way of thinking if it is to survive!" (Albert Einstein)
 We can now deduce the most simple science theory of reality - the wave structure of matter in space. By understanding how we and everything around us are interconnected
in Space we can then deduce solutions to the fundamental problems of human knowledge in physics, philosophy, metaphysics, theology, education, health, evolution and ecology, politics and society.
We can now deduce the most simple science theory of reality - the wave structure of matter in space. By understanding how we and everything around us are interconnected
in Space we can then deduce solutions to the fundamental problems of human knowledge in physics, philosophy, metaphysics, theology, education, health, evolution and ecology, politics and society.
This is the profound new way of thinking that Einstein
realised, that we exist as spatially extended structures of the universe - the discrete and separate body an illusion. This simply confirms the
intuitions of the ancient philosophers and mystics.
Given the current censorship in physics / philosophy of science journals (based on the standard model of particle physics / big bang cosmology) the internet is the best hope for getting new knowledge
known to the world. But that depends on you, the people who care about science and society, realise the importance of truth and reality.
It is Easy to Help!
Just click on the Social Network links at top of page, or copy a nice image or quote you like and share it. We have a wonderful collection of knowledge from the greatest minds in human history, so people will appreciate your contributions. In doing this you will help a new generation of scientists see that there is a simple sensible explanation of physical reality (One Substance, One Law) - the source of truth and wisdom, the only cure for the madness of man! Thanks! Geoff Haselhurst (Updated May, 2025)
A new scientific truth does not triumph by convincing its opponents and making them see the light, but rather because its opponents eventually die, and a new generation grows up that is familiar with it. (Max Planck, 1920)
"All that is necessary for evil to succeed is for good people to do nothing."
(Edmund Burke)
"In a time of universal deceit - telling the truth is a revolutionary act."
(George Orwell)
"Hell is Truth Seen Too Late."
(Thomas Hobbes)
Legal Disclaimer and Privacy Policy












